There are a variety of methods employed in extracting essential oils from aromatic plants, however, the process of distillation is the main method for extracting the aromatic parts of plants.
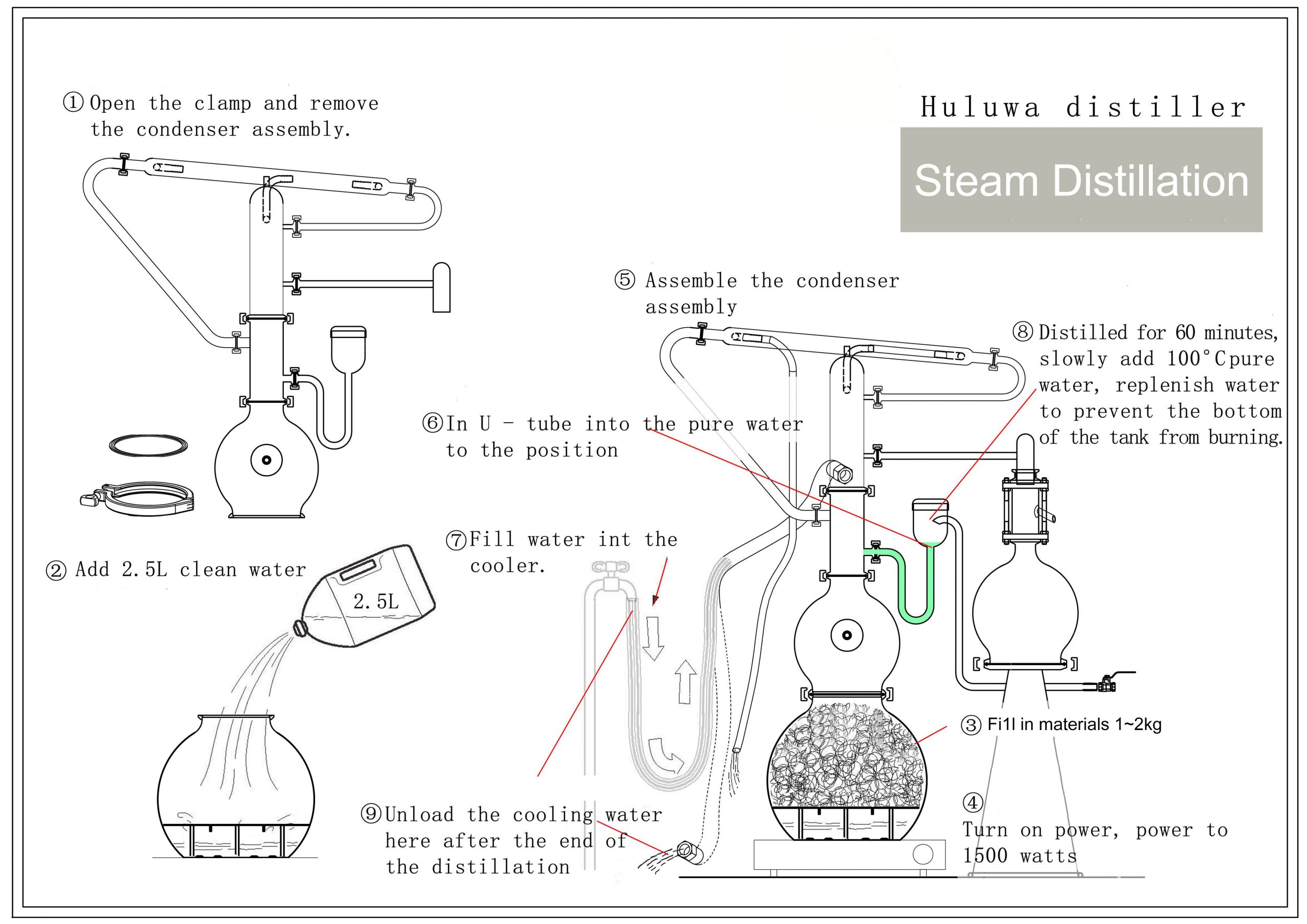

Steam Distillation
The plant material (called the ‘charge’) is placed in a still and then hot steam is passed through it. The heat breaks open the essential oil storage chambers within the charge, releasing the oil into the steam (remember, essential oils are volatile). The steam/oil rise to the top of the still where they enter a condenser – basically, a long spiral pipe surrounded by cold water – which condenses the steam back into its water form. At the end of the condenser, the water and essential oil are collected in a receiver, usually called a ‘Florentine flask’. This specially designed container has two outflows in view of the fact that oil and water don’t mix, i.e. the solution separates into essential oil and hydrolat. Essential oils are usually lighter than water and so will float above it.
Once collected, the lower outflow in the Florentine flask, allows the hydrolat to be drawn off, and since more of this is produced than oil, the process needs to be carried out during distillation to prevent the flask from overflowing.
However, before distillation was invented, ‘expression’ was one of the main methods used to extract essential oils from plants.
Expression
This method was used to extract essences from the citrus family. The rinds of the fruit were literally squeezed by hand until the oil glands burst releasing the oil which was then collected in a sponge. Once saturated, the sponge would be squeezed out into a container.
Nowadays, this method of extraction is carried out using machinery, rather than by hand.
Enfleurage
Another historical method used for extracting essential oil from flowers is the process of ‘enfleurage’.
Aromatic flower heads are placed on sheets of glass called ‘chassis’ which have been covered with purified fat. The fat absorbs the essential oils from the flowers and, once exhausted, the flowers are removed and replaced with fresh. This process is repeated many times until the fat is saturated with essential oil. The resultant compound is called a ‘pomade’. The pomade is then dissolved in alcohol. Fat is insoluble in alcohol but essential oil readily dissolves into it. The resultant liquid is then carefully heated and as the alcohol evaporates first, the pure essential oil is left behind.
Maceration
This process is similar to enfleurage and is a method by which one can still make essential oil at home in a ready diluted state.
The flowers or leaves are crushed to rupture some of the oil glands or cells and then put into a vegetable oil which is kept warm. The vegetable oil absorbs the essential oil and the plant material is strained off. Fresh plant material is then added to the re-warmed carrier and this process is repeated until the fat or vegetable oil is concentrated enough.
Solvent Extraction
This is one of the most modern methods of extraction and is mainly used for costly and delicate flower oils, such as jasmine, rose and tuberose, etc. Technically, products of solvent extraction are not essential oils, but are more accurately referred to as ‘absolutes’.
Unfortunately though, solvent extraction does involve the use of harsh chemicals, residues of which will inevitably remain within the aromatic absolute, resulting in potential skin irritation. As such, absolutes are not considered to be suitable for use in massage.
A simplified version of the solvent extraction process is as follows:
- Firstly, the flowers are covered with a chemical solvent, which absorbs the essential oil. The mixture of solvent and essential oil is referred to as an ‘extract’.
- The next stage involves the removal of the solvent and this is achieved by distilling the extract at a low pressure, thereby reducing the boiling point of the solvent, so that only gentle heat is required to remove it, leaving behind the aromatic molecules.
- The concentrated extract is then cooled causing it to solidify to a waxy consistency – at this point it is referred to as a ‘concrete’.
- In order to remove the unwanted wax, the concrete is ‘washed’ and warmed in alcohol which causes the oils to dissolve into it.
- The alcohol mixture is then chilled to separate out any remaining waxes, filtered and the alcohol removed by vacuum distillation at the lowest possible temperature. This final product is then referred to as an ‘absolute’.
Carbon Dioxide (CO2) Extraction
CO2 extraction is a relatively recent development over the last few decades. It produces oils that are very pure and with a unique quality, such that they can differ greatly from steam distilled essential oils. Other advantages include the fact that CO2 is inert which means that it does not react chemically with the oil being extracted, it is non-toxic, colourless and odourless, temperatures are kept very low, so thermally labile compounds do not suffer damage; there are no ‘still’ notes and more top notes; the true natural odour and flavour characteristics are retained.
Basically, carbon dioxide can take the form of either a liquid or gaseous state depending upon the atmospheric pressure and temperature it is subjected to. At above 33°C and over 200 atmospheres (i.e., 200 times that of regular atmospheric pressure), CO2 reaches a ‘hypercritical state’, i.e. it is too hot to be a conventional liquid, and too pressurised to be a conventional gas, i.e. it is at the tipping point between. As such, it can be widely dispersed amongst the charge and have solvent properties. Hypercritical CO2 extraction allows for the production of oils at low temperatures and very quickly (i.e., just a few minutes), with no chemical residue attached to the final product. Once extraction is complete, the pressure is released and the carbon dioxide returns to its gaseous state, leaving behind the whole oil.
Because the extraction process takes place in a completely sealed chamber, the whole oil is recovered, including the most volatile and fragile components. However, this has a downside, in that the concentration of pesticide residues, from the original plant material, are considerably greater than the values obtained by other more conventional methods of extraction. CO2 extraction really does recover everything from the plant material.

